TOP > News > Yolk-Shell Nanocrystals with Movable Gold Yolk: Next Generation of Photocatalysts: Assoc. Prof. Tso-Fu Mark Chang
News
Owing to their unique permeable, hollow shell structures with inner, movable cores, yolk-shell nanocrystals are suitable for a wide variety of applications. Yolk-shell nanocrystals consisting of a gold core with various semiconductor shells have been developed by Tokyo Tech researchers, using a novel sequential ion-exchange process. These metal-semiconductor yolk-shell nanocrystals can serve as highly effective photocatalysts for many applications.
Yolk-shell nanocrystals are unique materials with fascinating structural properties, such as a permeable shell, interior void space, and movable yolk. These nanocrystals are suitable for a variety of applications, depending on the choice of materials used for their fabrication.
For example, if the inner surface of their shells are reflective, yolk-shell nanocrystals can make for a reliable photovoltaic device. A mobile core can can act as a stirrer, capable of mixing solutions held within the shell. The inner and outer surfaces of the shell provide plenty of active sites for reactions, and the yolk-shell structure’s fascinating properties (a result of electronic interactions and charge-transfer between the surfaces of the structure) make these nanocrystals ideal for photocatalysis applications. Understandably, yolk-shell nanocrystals have earned the attention of researchers worldwide.
Now, in a collaborative study published in ACS Applied Nano Materials, which was also selected as the ACS Editors’ Choice, an international research team led by Associate Professor Tso-Fu Mark Chang and Assistant Professor Chun-Yi Chen at Tokyo Institute of Technology (Tokyo Tech) and Professor Yung-Jung Hsu at the National Yang Ming Chiao Tung University in Taiwan have developed several yolk-shell structures containing a metallic gold (Au) yolk with various semiconductor shells. Such structures have risen in popularity worldwide because of their fascinating properties, owing to their Au cores.
“Yolk-shell nanocrystals comprising of a metal yolk and semiconductor shells are particularly interesting because they can be geared to mass transport-related utilizations, for example, photocatalysis,” says Professor Chen.
To make the nanocrystals, the researchers employed a sequential ion-exchange process. The procedure involves delicate sulfidation on an Au@Cu2O core-shell nanocrystal template (where Au contributes to the core, and Cu2O to the shell formation), followed by a kinetically controlled cation exchange reaction that enables conversion of the shell composition (i.e., Cu2O) into various metal sulfides, which are semiconductors. Four representative yolk-shell nanocrystal samples, including Au@Cu7S4, Au@CdS, Au@ZnS, and Au@Ni3S4, were synthesized for investigation in this way, as shown in Figure 1.
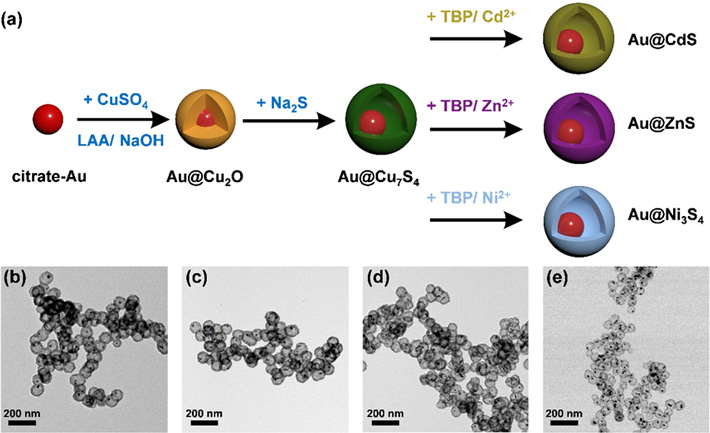
Figure 1. (a) Schematic depiction of the synthetic procedure for Au@Cu7S4, Au@CdS, Au@ZnS, and Au@Ni3S4.
(b-e) shows the corresponding TEM images.
The synthesis of yolk-shell nanostructures involves sulfidation on an Au@Cu2O core-shell nanocrystal template to convert the shell composition to various metal sulphides.
Sone-Chang Lab(Advanced Materials Research Core)
http://www.ames.pi.titech.ac.jp/